Section: Scientific Foundations
Biomechanical Modeling
Biomechanical modeling of solid structures
Soft tissue modeling holds a very important place in medical simulation. A large part of the realism of a simulation, in particular for surgery or laparoscopy simulation, relies upon the ability to describe soft tissue response during the simulated intervention. Several approaches have been proposed over the past ten years to model soft-tissue deformation in real-time (mainly for solid organs), usually based on elasticity theory and a finite element approach to solve the equations. We were among the first to propose such an approach [24] , [27] using different computational strategies. Although significant improvements were obtained later on (for instance with the use of co-rotational methods to handle geometrical non-linearities) these works remain of limited clinical use as they rely on linearized constitutive laws.
|
An important part of our research is dedicated to the development of new, more accurate models that remain compatible with real-time computation. Such advanced models will not only permit to increase the realism of future training systems, but they will act as a bridge toward the development of patient-specific preoperative planning as well as augmented reality tools for the operating room. Yet, patient-specific planning or per-operative guidance also requires the models to be parametrized with patient-specific biomechanical data. Very little work has been done in this area, in particular when tissue properties need to be measured in vivo non-invasively. New imaging techniques, such as Ultrasound Elastography or Magnetic Resonance Elastography, could be used to this end [23] . We are currently studying the impact of parametrized patient-specific models of the liver in the context of the PASSPORT european project. This will be used to provide information about the deformation, tissue stiffness and tumor location, for various liver pathologies.
Biomechanical modeling of hollow structures
A large number of anatomical structures in the human body are vascularized (brain, liver, heart, kidneys, ...) and recent interventions (such as interventional radiology) rely on the vascular network as a therapeutical pathway. It is therefore essential to model the shape and deformable behavior of blood vessels. This will be done at two levels. Global deformation of a vascular network: we have demonstrated previously [9] that we could recover the shape of thousands of vessels from medical images by extracting the centerline of each vessel (see Figure 2). The resulting vascular skeleton can be modeled as a deformable (tree) structure which can capture the global aspects of the deformation. More local deformations can then be described by considering now the actual local shape of the vessel. Other structures such as aneurysms, the colon or stomach can also benefit from being modeled as deformable structures. For this we will rely on shell or thin plate theory. We have recently obtained very encouraging results in the context of the Ph.D. thesis of Olivier Comas [26] . Such local and global models of hollow structures will be particularly relevant for planning coil deployment or stent placement, but also in the context of a new laparoscopic technique called NOTES which uses a combination of a flexible endoscope and flexible instruments. Obtaining patient-specific models of vascular structures and associated pathologies remains a challenge from an image processing stand point, and this challenge is even greater once we require these models to be adapted to complex computational strategies. To this extend we will pursue our collaboration with the MAGRIT team at Inria (through a PhD thesis starting in January 2010) and the Massachusetts General Hospital in Boston.
Blood Flow Simulation
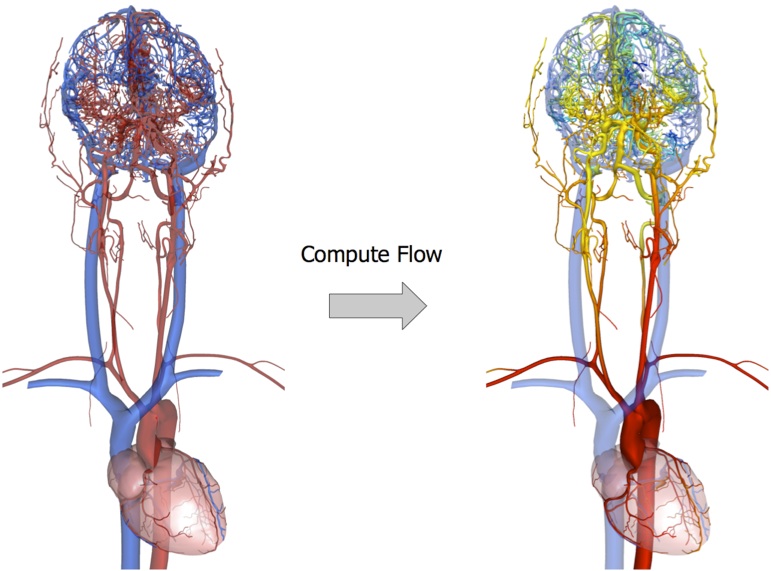
Beyond biomechanical modeling of soft tissues, an essential component of a simulation is the modeling of the functional interactions occurring between the different elements of the anatomy. This involves for instance modeling physiological flows (blood flow, air flow within the lungs...). We particularly plan to study the problem of fluid flow in the context of vascular interventions, such as the simulation of three-dimensional turbulent flow around aneurysms to better model coil embolization procedures. Blood flow dynamics is starting to play an increasingly important role in the assessment of vascular pathologies, as well as in the evaluation of pre- and post-operative status. While angiography has been an integral part of interventional radiology procedures for years, it is only recently that detailed analysis of blood flow patterns has been studied as a mean to assess complex procedures, such as coil deployment. A few studies have focused on aneurysm-related hemodynamics before and after endovascular coil embolization. Groden et al. [31] constructed a simple geometrical model to approximate an actual aneurysm, and evaluated the impact of different levels of coil packing on the flow and wall pressure by solving Navier-Stokes equations, while Kakalis et al. [33] relied on patient-specific data to get more realistic flow patterns, and modeled the coiled aneurysm as a porous medium. As these studies aimed at accurate Computational Fluid Dynamics simulation, they rely on commercial software, and the computation times (dozens of hours in general) are incompatible with interactive simulation or even clinical practice. Generally speaking, accuracy and efficiency are two significant pursuits in numerical calculation, but unfortunately very often contradictory.
|
With the Ph.D. thesis of Yiyi Wei, we have recently started the development of a new technique for accurately computing, in near real-time, the flow of blood within an aneurysm, as well as the interaction between blood and coils. In this approach we rely on the Discrete Exterior Calculus method to obtain an ideal trade-off between accuracy and computational efficiency. Although still at an early stage, these results show that our approach can accurately capture the main characteristics of the complex blood flow patterns in and around an aneurism. The model also takes into account the influence of the coil on the blood flow within the aneurysm. The main difference between our approach and many other work done by internationally renowned teams (such as REO team at Inria or the Computer Vision Laboratory at ETH) comes from the importance we place in the computational efficiency of the method. To some extent our approach is similar to what has been done to obtain real-time finite element methods. We are essentially trying to capture the key characteristics of the behavior for a particular application. This is well illustrated by the work we started on flow modeling, which received an award in September 2009 at the selective conference on Medical Image Computing and Computer Assisted Interventions [10] . We will pursue this direction to accurately model the local flow in a closed domain (blood vessel, aneurysm ventricle, ...) and combine it with some of our previous work describing laminar flow across a large number of vessels [38] in order to define boundary conditions for the three-dimensional model.